NCKU-CYU Collaboration Reveals Hidden Danger of Nanoplastics to Gut Microbiota
Written & Image credit to Wei‑Hsuan Hsu
Scientists have become increasingly concerned about the negative impacts of microplastics on human health. However, due to their varied compositions and sizes, the exact mechanisms of damage—especially for nanoplastics—remain unclear. Associate Professor Wei‑Hsuan Hsu from Department of Food Safety/Hygiene and Risk Management in NCKU, together with Assistant Professor Bao‑Hong Lee from the Department of Horticulture at National Chiayi University (NCYU), have discovered that nanoplastics can “hijack” the signaling systems between gut cells and bacteria. This process alters the symbiotic relationship between microbiota and their host, disrupting microbial balance and impairing gut barrier function, ultimately leading to leaky gut. These groundbreaking findings have attracted international attention and have been published in the prestigious journal Nature Communications under the title:Polystyrene nanoplastics disrupt the intestinal microenvironment by altering bacteria-host interactions through extracellular vesicle-delivered microRNAs

Associate Professor Wei‑Hsuan Hsu from NCKU and Assistant Professor Bao‑Hong Lee from NCYU highlight how nanoplastics can hijack signaling pathways between gut cells and bacteria, altering host‑microbiome symbiosis, impairing barrier function, and causing leaky gut. This study has garnered international acclaim and is published in the top-tier journal Nature Communications.
The research team used mice models to uncover, for the first time, a novel mechanism by which nanoplastics harm gut health—marking a milestone in plastic pollution risk assessment. In just three weeks since online publication, the paper has been accessed or downloaded nearly 20,000 times. It was selected as a Highlight Article, and its Altmetric score ranks it in the top 1% among over 200,000 concurrent publications.
Previously, in a hydroponic vegetable study, the team also found that although nanoplastics in water do not affect lettuce growth directly, they promote the growth of specific bacteria in the system. These bacteria secrete extracellular vesicles that suppress the plant’s antioxidant defenses and growth mechanisms, eventually causing wilting. The findings were published in the Journal of Hazardous Materials under the title:Nanoplastics indirectly compromise lettuce growth in hydroponic systems via microbial extracellular vesicles derived from Curvibacter fontanus
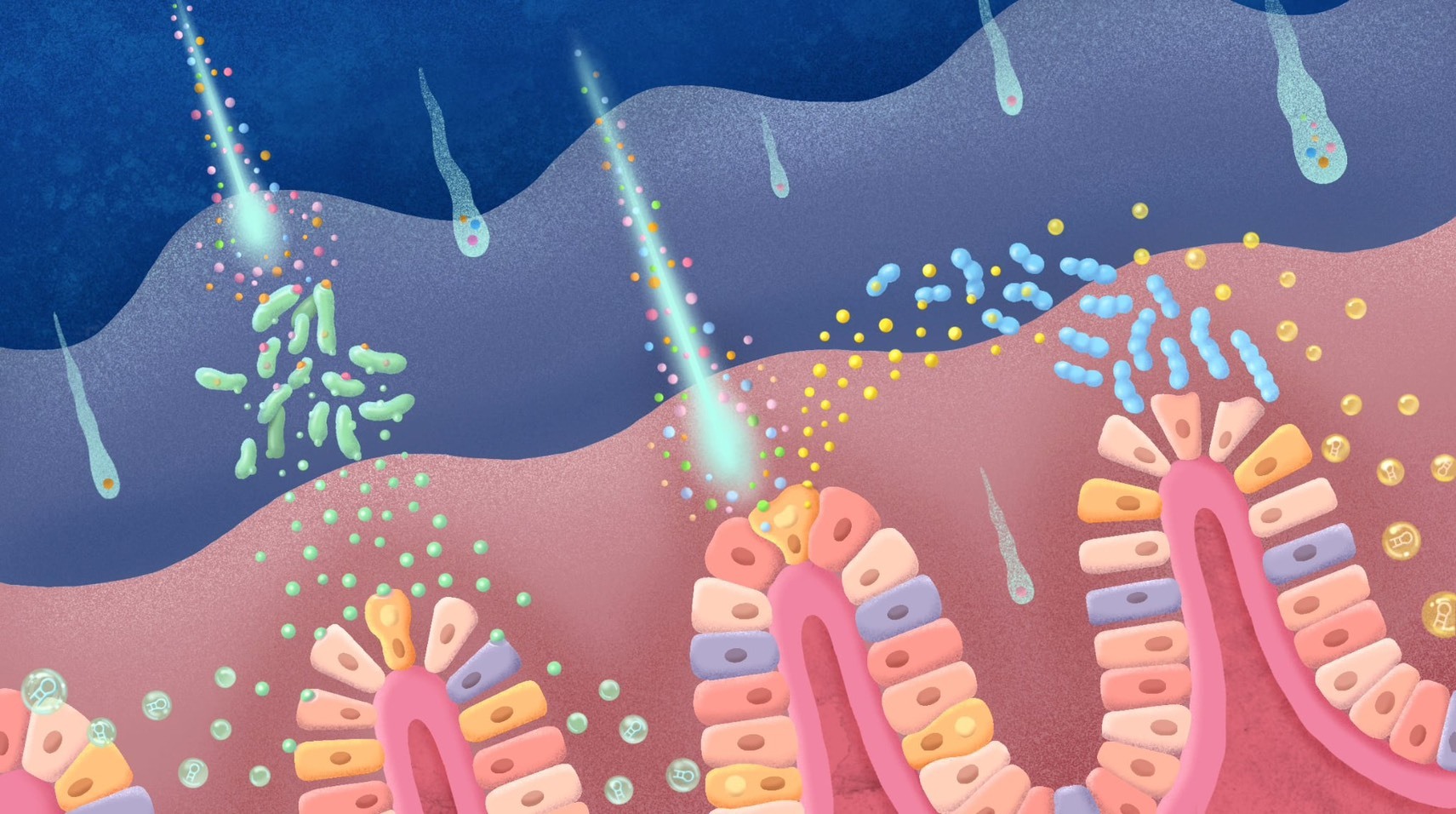
Nanoplastic particles—with the force of a comet—pierce the gut’s protective barrier, attacking both gut bacteria and intestinal cells. Triggered bacteria and cells then release extracellular vesicles that weaken cell‑cell junctions and alter gut microbiota, resulting in leaky gut.
Associate Professor Hsu explains that microscopic plastics are a major global concern, polluting oceans and soils—and now demonstrated to impact crop plants and animal health. She notes that while particles above ~150 microns are typically expelled from the body, nanoplastics (which are 1 µm = 1,000 nm) may accumulate in internal organs and potentially breach natural barriers like those of the gut and brain. This could allow inflammatory agents or bacteria to enter the bloodstream, yet the limited literature so far hinders comprehensive understanding.
Extracellular vesicles—tiny cell‑secreted vesicles—act as messengers, facilitating intercellular communication. The team has long studied their role in cross‑kingdom signaling (e.g., between cells and microbes).
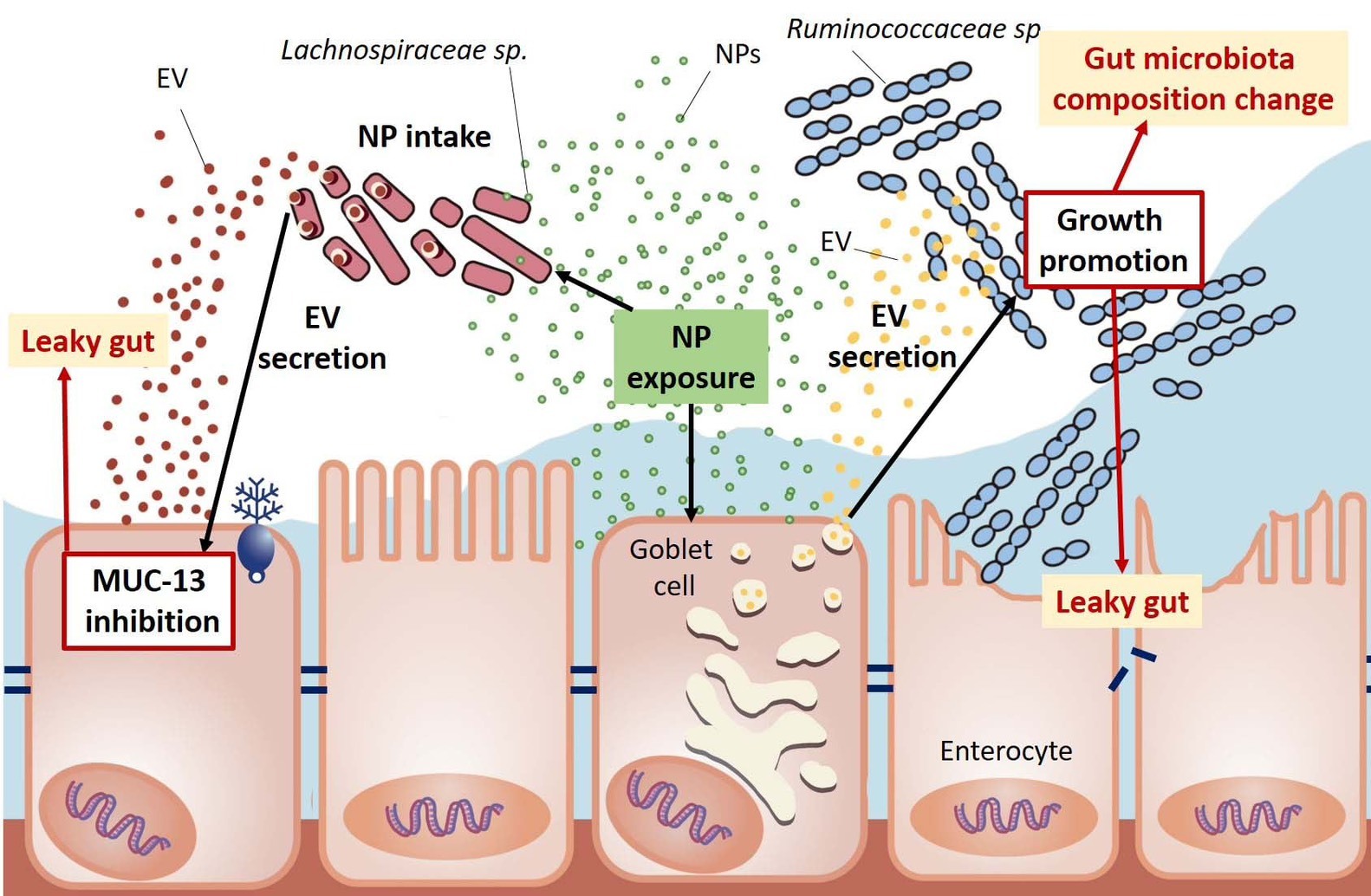
Nanoplastics disrupt the balance of gut microbiota and impair intestinal barrier function by stimulating gut bacteria and host cells to release extracellular vesicles.
In their experiments, mice were fed nanoplastics for 12 weeks. The findings revealed that nanoplastics stimulated gut cells and certain bacteria to release extracellular vesicles containing microRNAs. These microRNAs reprogram gut functions by altering the microbial balance—reducing beneficial bacteria, promoting the growth of harmful species, and compromising the integrity of the gut barrier. This study is the first to show that nanoplastics can indirectly harm living organisms, including both plants and animals, through mechanisms involving extracellular vesicle communication.
While this research decodes the molecular mechanism of nanoplastic interference in gut microbiota, differences between mice and humans still exist. Therefore, the direct health implications for humans remain to be evaluated. The team has established a human gut‑mimicking microbial dynamic system to assess the effects of nanoplastics and other substances on human gut microbiome.
When the gut mucosa is damaged, it becomes inflamed and gaps form between intestinal cells. This allows unwanted substances such as undigested food, bacteria, and toxins to leak into the bloodstream, potentially leading to a condition called leaky gut syndrome.

The collaborative team from NCKU and NCYU, dedicated to exploring microbial extracellular vesicle function, received the 2024 Future Tech Award. Fourth from the right is Associate Professor Hsu; third from the left is Assistant Professor Lee, joined by their lab members.
Professor Hsu emphasizes that this research not only broadens our understanding of nanoplastic toxicity but also highlights extracellular vesicles as central mediators in communication between microbiota and their host. She believes this discovery could pave the way for innovative treatments, including targeting vesicle production or restoring disrupted microRNA communication, to mitigate the harmful impact of nanoplastics.
Assistant Professor Lee notes that this research highlights the regulatory role of extracellular vesicles in host–microbiota interactions, a field of growing scientific interest. Their goal is to identify novel therapeutic targets by harnessing the functions of microbial extracellular vesicles.
The research received support from the NSTC’s Outstanding Young Scholar Program and funding from the Ministry of Health and Welfare. Its successful completion showcases Taiwan's strong innovation capacity. Given the global boom in extracellular vesicle research, the team hopes Taiwan will invest more in this field to gain international visibility and leadership.
Provider:
NCKU News Center
Date:
2025-07-08