Solving the Mystery of Alzheimer's Disease and Dementia: NCKU Research Team Confirms New Risk Factor WWOX


As medical communities around the world strive to unlock the mystery behind Alzheimer's disease (AD) and dementia, the National Institutes of Health (NIH), USA, recently announced five new genes as risk factors for AD. Among these genes, WWOX gene was first discovered by a research team led by Distinguished Professor Nan-Shan Chang of the Institute of Molecular Medicine at the National Cheng Kung University (NCKU) in Tainan, Taiwan.
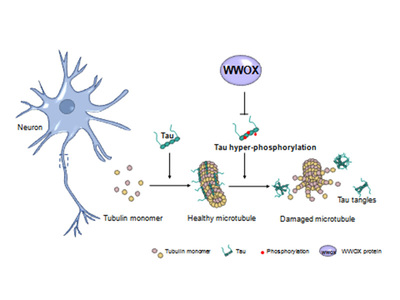
Prior to the NIH’s press release, Chang’s team has been working on WWOX in the areas of both cancer and AD for more than 19 years. His team and coworkers’ discovery is that a reduction of WWOX protein or an aberrant alteration of WWOX protein function in the brain is associated with the AD progression with age.
Chang’s team also discovered a 31-amino-acid small protein or a short peptide, named Zfra (zinc finger like protein that regulates apoptosis) in 2005. Zfra was further truncated or reduced to 7 amino acids, dubbed “Tiny Z”. Both the full-length Zfra and Tiny Z peptides can be synthesized chemically and have recently been reported to mitigate the AD symptoms. Tiny Z restores memory loss in a mouse model for age-related development of AD-like symptoms. In light of this finding, Tiny Z is considered of therapeutic potential for treating AD. The patent for using Zfra and Tiny Z in AD treatment has been granted by the US Patent and Trading Office early in May 2019. This is in addition to the globally patented function of Zfra and Tiny Z in cancer treatment.
In 2011, 474 genome scientists in 300 top genetic research teams around the world, mainly based in the US and Europe, began collaborating to test and study genetic variants associated with AD in order to identify the pathogenic genes and the underlying mechanisms for the onset and progression of the disease. The genetic variants of 94,437 patients with late-onset AD were studied and analyzed, and the latest results were published in Nature Genetics at the end of February, 2019. Aside from the 20 genes already discovered to be risk factors for AD, the five new genes are IQCK, ACE, ADAM10, ADAMTS 1, and WWOX.
Among these genes, WWOX has received the most attention. Scientists have been working on the cancer inhibitory effects of WWOX since 2000. NCKU was the first in the world to examine the physiological role of WWOX in the maintenance of neural functions. For more than a decade, NCKU research teams have proven that a reduction in WWOX protein expression causes a protein-aggregation chain reaction leading to mitochondrial dysfunction for neuronal cell degeneration and death and gradual development of dementia. Mitochondria are known as the powerhouse of the cell for energy generation, and yet the ghost house due to the presence of numerous killer proteins.
While studying WWOX, the NCKU research teams also determined that Zfra and Tiny Z increase WWOX stability, so as to protect and sustain neuronal or brain cell survival. Both Zfra and Tiny Z are made from chemical synthesis and then subjected to purification for therapeutic purposes. The chemical safety and drug stability have yet to be tested in large-size animals in the preclinical trials. The NCKU teams call for industrial investment for preclinical testings, prior to FDA approval for clinical trials in AD patients and other types of neurodegeneration, as well as cancer.
Vice Dean Yu-Min Kuo of the College of Medicine at NCKU pointed out that all of the information regarding WWOX published in the study in Nature Genetics came from the research work from Chun-I Sze and Nan-Shan Chang laboratories and other NCKU teams. The most crucial discovery regarding the connection between WWOX and AD was established in 2004. The report demonstrated that reduction of WWOX protein and a site-specific reduction in amino acid phosphorylation in white Caucasian AD patients of 70 to 100 years old.
Later in 2015, Chang and his graduate student Jean Chang reported that in the middle-aged normal individuals of 40 to 70, there is a series of brain proteins which may undergo polymerization or aggregation that leads to the generation of tau and amyloid beta aggregation. Both tau and amyloid beta aggregation are hallmarks of AD as both protein aggregates are abundant in the brain hippocampus of old-aged AD patients. In 2017, the NCKU teams including Yu-Min Kuo, Sze and Chang laboratories, reported the function of Zfra and Tiny Z in mitigating the AD symptoms in the so-called triple transgenic mice during their aging process. These mice develop AD-like symptoms such as memory loss when they grow old.
WWOX (WW domain-containing oxidoreductase) was first discovered and named by Nan-Shan Chang in 2000 during his tenure at the Guthrie Research Institute, Sayre, Pennsylvania, USA. One of the NCKU research team members, Professor Chun-I Sze of the Department of Cell Biology and Anatomy at NCKU, “WWOX being listed as a risk factor of Alzheimer's disease by the international community is a testimony to the research strength of the NCKU research teams. Nan-Shan Chang said, “WWOX is my 19-year-old child, and its appearance in CNN News was extremely exciting.”
Professor Shur-Tzu Chen was the pioneer who started studying WWOX in neuroscience in 2003. She was at the Department of Cell Biology and Anatomy at NCKU. When Nan-Shan Chang relocated to NCKU from the USA in 2007, he continued his research in WWOX on cancer and AD. On campus team efforts have been involving: Professors Shur-Tzu Chen, Chun-I Sze, and Yu-Min Kuo of the Department of Cell Biology and Anatomy, Professor Li-Jin Hsu of the Department of Medical Laboratory Science and Biotechnology, and Professor Pei-Jung Lu from the Institute of Clinical Medicine. Under Chang’s guidance, graduate student Jean-Yun Chang at the Institute of Molecular Medicine determined that a unique protein aggregation mechanism in the brain of middle-aged individuals, which is associated with old-age dementia and published her paper in 2015. NCKU teams have produced 52 out of 468 WWOX-related research papers currently listed in the PubMed database among global scientists. Chang put it, “The momentum to work on WWOX in AD is expected to raise rapidly. Thanks for the global efforts by geneticists.”
The number of people living with dementia is rising swiftly, and Alzheimer's disease is one of the most common types of dementia. Aside from memory loss, Alzheimer's disease also affects the speech, sense of space, judgment, attention, and abstract thinking abilities of patients and may even result in personality changes, delusions, or hallucinations, which severely impact their social life and work abilities. Alzheimer's Disease International estimated that approximately 50 million individuals were living with dementia around the world in 2018 and that this number would increase to 152 million in 2050. Based on demographic statistics from the end of December, 2018, the Ministry of the Interior estimates that there are roughly more than 282,000 people with dementia in Taiwan, which is equal to 1 out of every 84 people.
Prior to the NIH’s press release, Chang’s team has been working on WWOX in the areas of both cancer and AD for more than 19 years. His team and coworkers’ discovery is that a reduction of WWOX protein or an aberrant alteration of WWOX protein function in the brain is associated with the AD progression with age.
Chang’s team also discovered a 31-amino-acid small protein or a short peptide, named Zfra (zinc finger like protein that regulates apoptosis) in 2005. Zfra was further truncated or reduced to 7 amino acids, dubbed “Tiny Z”. Both the full-length Zfra and Tiny Z peptides can be synthesized chemically and have recently been reported to mitigate the AD symptoms. Tiny Z restores memory loss in a mouse model for age-related development of AD-like symptoms. In light of this finding, Tiny Z is considered of therapeutic potential for treating AD. The patent for using Zfra and Tiny Z in AD treatment has been granted by the US Patent and Trading Office early in May 2019. This is in addition to the globally patented function of Zfra and Tiny Z in cancer treatment.
In 2011, 474 genome scientists in 300 top genetic research teams around the world, mainly based in the US and Europe, began collaborating to test and study genetic variants associated with AD in order to identify the pathogenic genes and the underlying mechanisms for the onset and progression of the disease. The genetic variants of 94,437 patients with late-onset AD were studied and analyzed, and the latest results were published in Nature Genetics at the end of February, 2019. Aside from the 20 genes already discovered to be risk factors for AD, the five new genes are IQCK, ACE, ADAM10, ADAMTS 1, and WWOX.
Among these genes, WWOX has received the most attention. Scientists have been working on the cancer inhibitory effects of WWOX since 2000. NCKU was the first in the world to examine the physiological role of WWOX in the maintenance of neural functions. For more than a decade, NCKU research teams have proven that a reduction in WWOX protein expression causes a protein-aggregation chain reaction leading to mitochondrial dysfunction for neuronal cell degeneration and death and gradual development of dementia. Mitochondria are known as the powerhouse of the cell for energy generation, and yet the ghost house due to the presence of numerous killer proteins.
While studying WWOX, the NCKU research teams also determined that Zfra and Tiny Z increase WWOX stability, so as to protect and sustain neuronal or brain cell survival. Both Zfra and Tiny Z are made from chemical synthesis and then subjected to purification for therapeutic purposes. The chemical safety and drug stability have yet to be tested in large-size animals in the preclinical trials. The NCKU teams call for industrial investment for preclinical testings, prior to FDA approval for clinical trials in AD patients and other types of neurodegeneration, as well as cancer.
Vice Dean Yu-Min Kuo of the College of Medicine at NCKU pointed out that all of the information regarding WWOX published in the study in Nature Genetics came from the research work from Chun-I Sze and Nan-Shan Chang laboratories and other NCKU teams. The most crucial discovery regarding the connection between WWOX and AD was established in 2004. The report demonstrated that reduction of WWOX protein and a site-specific reduction in amino acid phosphorylation in white Caucasian AD patients of 70 to 100 years old.
Later in 2015, Chang and his graduate student Jean Chang reported that in the middle-aged normal individuals of 40 to 70, there is a series of brain proteins which may undergo polymerization or aggregation that leads to the generation of tau and amyloid beta aggregation. Both tau and amyloid beta aggregation are hallmarks of AD as both protein aggregates are abundant in the brain hippocampus of old-aged AD patients. In 2017, the NCKU teams including Yu-Min Kuo, Sze and Chang laboratories, reported the function of Zfra and Tiny Z in mitigating the AD symptoms in the so-called triple transgenic mice during their aging process. These mice develop AD-like symptoms such as memory loss when they grow old.
WWOX (WW domain-containing oxidoreductase) was first discovered and named by Nan-Shan Chang in 2000 during his tenure at the Guthrie Research Institute, Sayre, Pennsylvania, USA. One of the NCKU research team members, Professor Chun-I Sze of the Department of Cell Biology and Anatomy at NCKU, “WWOX being listed as a risk factor of Alzheimer's disease by the international community is a testimony to the research strength of the NCKU research teams. Nan-Shan Chang said, “WWOX is my 19-year-old child, and its appearance in CNN News was extremely exciting.”
Professor Shur-Tzu Chen was the pioneer who started studying WWOX in neuroscience in 2003. She was at the Department of Cell Biology and Anatomy at NCKU. When Nan-Shan Chang relocated to NCKU from the USA in 2007, he continued his research in WWOX on cancer and AD. On campus team efforts have been involving: Professors Shur-Tzu Chen, Chun-I Sze, and Yu-Min Kuo of the Department of Cell Biology and Anatomy, Professor Li-Jin Hsu of the Department of Medical Laboratory Science and Biotechnology, and Professor Pei-Jung Lu from the Institute of Clinical Medicine. Under Chang’s guidance, graduate student Jean-Yun Chang at the Institute of Molecular Medicine determined that a unique protein aggregation mechanism in the brain of middle-aged individuals, which is associated with old-age dementia and published her paper in 2015. NCKU teams have produced 52 out of 468 WWOX-related research papers currently listed in the PubMed database among global scientists. Chang put it, “The momentum to work on WWOX in AD is expected to raise rapidly. Thanks for the global efforts by geneticists.”
The number of people living with dementia is rising swiftly, and Alzheimer's disease is one of the most common types of dementia. Aside from memory loss, Alzheimer's disease also affects the speech, sense of space, judgment, attention, and abstract thinking abilities of patients and may even result in personality changes, delusions, or hallucinations, which severely impact their social life and work abilities. Alzheimer's Disease International estimated that approximately 50 million individuals were living with dementia around the world in 2018 and that this number would increase to 152 million in 2050. Based on demographic statistics from the end of December, 2018, the Ministry of the Interior estimates that there are roughly more than 282,000 people with dementia in Taiwan, which is equal to 1 out of every 84 people.
Provider:
NCKU News Center
Date:
2019-05-08