International publication of easy-to-use vaccine protection test developed by Guan-Da Syu and his team at NCKU Biotech Department
Edited by & Image credit to News Center.
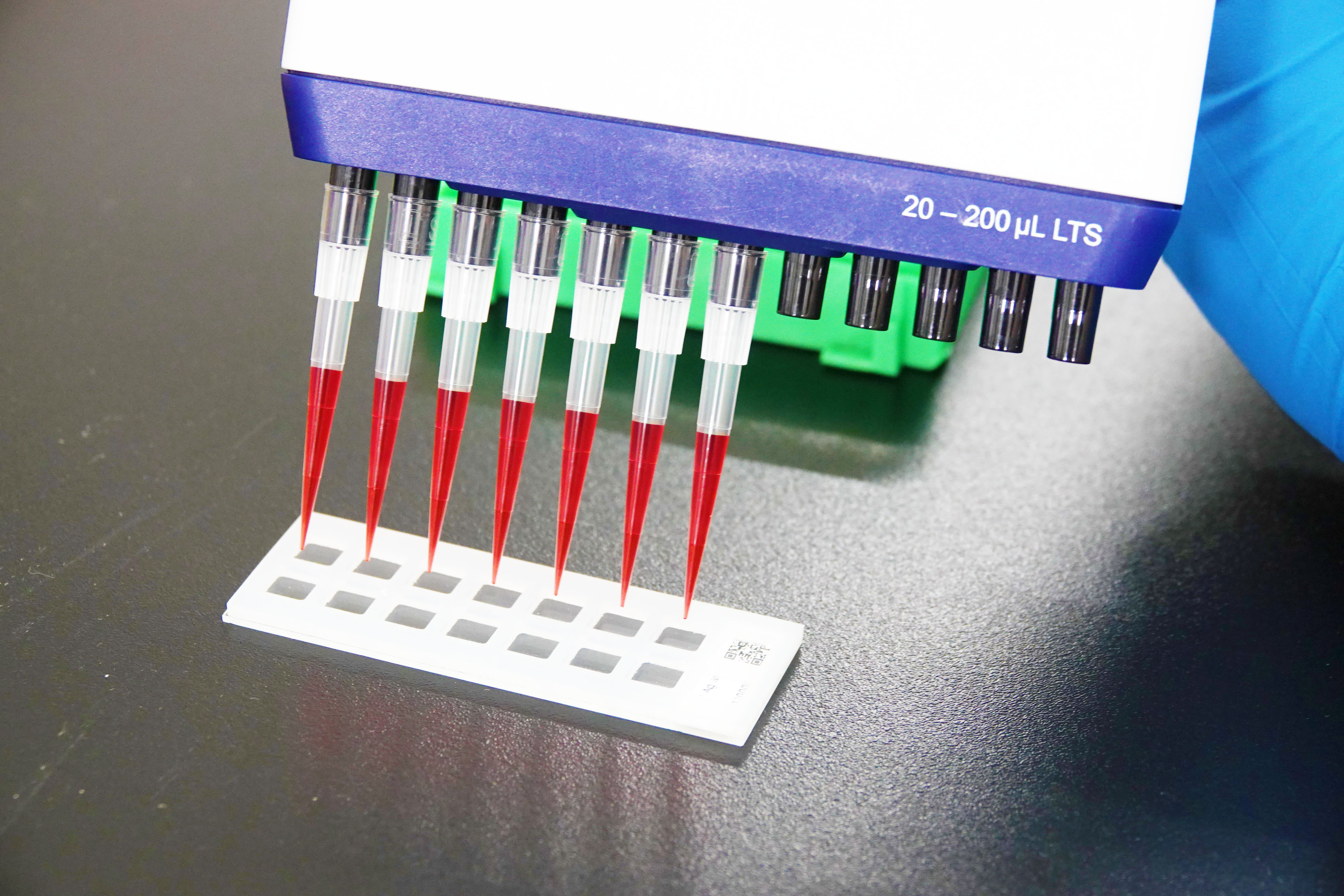
Group from NCKU develops novel high-throughput protein detection chip for coronavirus variants.
The recent resurgence of the COVID19 pandemic in Taiwan, coupled with menacing virus variants, has led many to ask the question: Do our vaccines provide enough protection? A team led by Assistant Professor Guan-Da Syu of the Department of Biotechnology and Bioindustry Sciences at NCKU has developed a novel high-throughput protein detection chip which can detect, using only a single drop of blood, whether one has neutralizing antibodies against variant strains of the coronavirus. The team also developed another protein chip that can be used to analyze the immune response of patients with mild or severe symptoms.
Detection and analysis of the immune characteristics of patients assists in injury classification or preventive drug administration. Results on both chips were recently published in the internationally-renowned journals Biosensors and Bioelectronics and Analytical Chemistry, not only enhancing the reputation of NCKU, but also making a significant contribution to the control and prevention of disease.
Guan-Da Syu pointed out that because the current vaccine is designed for the original Wuhan strain, its effectiveness against mutant strains remains unclear. Since everyone responds differently to the vaccine, the team developed a chip for the detection of resistance to multiple mutant strains. The high-throughput detection chip is the size of a fingertip and can simultaneously analyze blood samples for neutralization efficacy and antibody concentration against novel coronavirus variants in under one hour. The traditional approach to detection of neutralizing antibodies involves infecting blood cells with variants of the viruses. This process is both cumbersome and dangerous, and must be performed by specialist technicians.

Assistant Professor Guan-Da Syu of the Department of Biotechnology and Bioindustry Sciences, NCKU.
In contrast, NCKU’s detection technology simulates the combination of various viruses and receptors on the chip, which reduces the number of samples, as well as time and financial costs, required for the simultaneous analysis of multiple variants . The simplified experimental process also ensures the safety of operators. In addition to blood antibody testing, Guan-Da Syu’s team developed testing methods for affinity drugs such as protein drugs, antibody drugs, and small molecule drugs, which can comprehensively and rapidly evaluate their ability to fight off new coronavirus variants.

NCKU biotech group publishes ground-breaking paper.
Guan-Da Syu pointed out that due to the rapid evolution of the epidemic and the constant mutation of the virus, the most difficult task they faced was anticipating the different developments of the virus. In early 2020, when the coronavirus epidemic broke out, Guan-Da Syu devoted himself to COVID-19 detection and vaccine evaluation. With the help of Wen-Chien Ko, the vice president of the Affiliated Hospital of NCKU Medical College, Guan-Da Syu and the attending physician of the Pediatric Department of the Affiliated Hospital of NCKU Medical College were referred to Dr. Tzong-Shiann Ho of the Tainan Hospital, who serves as the director of pediatrics. There, they cooperated to apply academic research to clinical trials.
Key factors underlying Guan-Da Syu’s success include the support of his supervisors and colleagues and the efforts of his student team, as well as the rich academic resources of NCKU University and the medical capacity of the affiliated hospital.

Professor Guan-Da Syu (left) and student demonstrate use of the chip scanner.
A patent for the developed protein chip has been accepted in Taiwan and is currently under review in the United States. Guan-Da Syu reported, “We are currently discussing cooperation and technology transfer with manufacturers.” The team is also developing a portable scanner, to increase the convenience of application for clinics, telemedicine, and homes.
Provider:
News Center
Date:
2022-10-13